Phase II Container Laboratories
We will develop six (6) container mobile laboratories (one per partner NPHLs) which are able to culture bacterial pathogens at biological safety level 3 / 4. These laboratories can be loaded onto trucks, transported to the field and will thus be fully operational in remote, difficult to reach locations. They will be equipped with a negative pressure system compliant with BSL3 standards in order to host a state of the art culture facility to accurately diagnose bacterial diseases and define their antimicrobial resistance (AMR) profiles using culture/plate based phenotypic drug-susceptibility testing (DST). By also equipping the laboratories with next generation sequencing facilities suitable for field settings, the laboratory will be able to carry out genotypic DSTs as well. For an overview of a conceivable laboratory concept see figure 2.
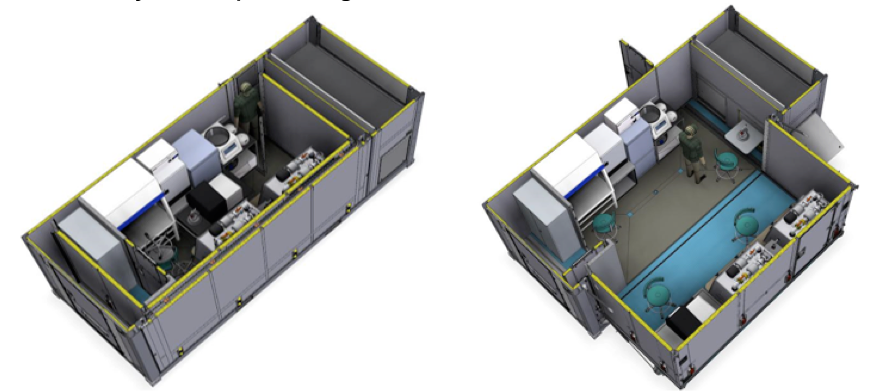
Figure 2: A potential setup of the Container Laboratory. The laboratory will be installed within an extendable 20ft container and contain a BSL-3 negative pressure system with separate gowning area and an airlock for sample import. It will be equipped with (i) Laminar flow hood and incubators for bacterial culture, (ii) a Bactec blood culture system, (iii) Minion sequencing and bioinformatics facility for genotypic resistance testing, (iv) Nucleic acid extraction robot, PCR Hood, PCR machine for pathogen identification, (v) glovebox for inactivation of BSL3/4 pathogens, (vi) blood chemistry facility, (vi) Centrifuges, fridge/freezer, autoclaves, (vii) ICT and LIMS connectivity, (viii) back-up generator to assure autonomous operation of laboratory
The laboratories will be equipped with essential bacterial culture equipment (incubators, blood culture systems), to isolate bacteria from blood, urine and stool and conduct antimicrobial resistance profiling. The bacterial work will follow guidelines outlined by the WHO Global Antimicrobial Resistance Surveillance System (GLASS) and a special emphasis will be put on the surveillance and AMR monitoring of priority AMR pathogens as defined by GLASS (see figure 3).
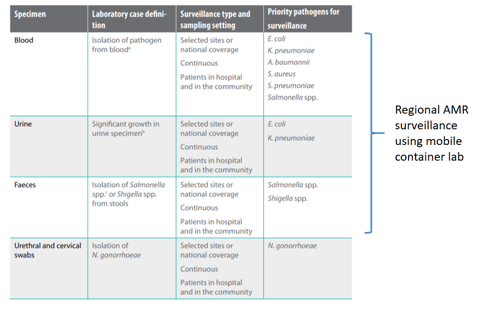
Figure 3: WHO Global Antimicrobial Resistance Surveillance System (GLASS) Priority specimens and pathogens for surveillance of AMR
Although the main focus of the labs is on AMR work, these container laboratories will also be equipped with a static BSL4 glovebox, which (in combination with the sequencing facility) will enable them to conduct epidemiological molecular analysis (to identify index cases in outbreaks) and identify novel pathogens as well as emerging strain variants of haemorrhagic fever viruses that would have been missed by classical diagnostic techniques. Therefore they will perfectly complement the already existing 9 modular laboratories procured during phase I of the project.
Through the use of an ICT platform with a laboratory information management system (LIMS), these mobile laboratories will have the capacity to handle large data sets and could be integrated with the existing LIMS system (established during phase I of the project ) and with national health reporting system of each country.